UDI: Unique Device Identification
1. What is UDI?
The UDI is a series of numeric or alphanumeric characters that is created through a globally accepted device identification and coding standard. It allows the unambiguous identification of a specific medical device on the market. The UDI contains two parts: an UDI-DI and an UDI-PI.
More about UDI, please refer to: FDA guidance.


- UDI-DI: A device identifier, a fixed code specific to a version or model of a medical device.
- UDI-PI: A production identifier, related to production data of the device, such as lot/batch number, expiry date, manufacturing date, etc.
2. UDI Parts (e.g. GS1)
(1) DI (Known as GTIN in UDI) include: Application Identifiers (AI), Packaging level indicator, Company prefix, Item reference number, Check digit.
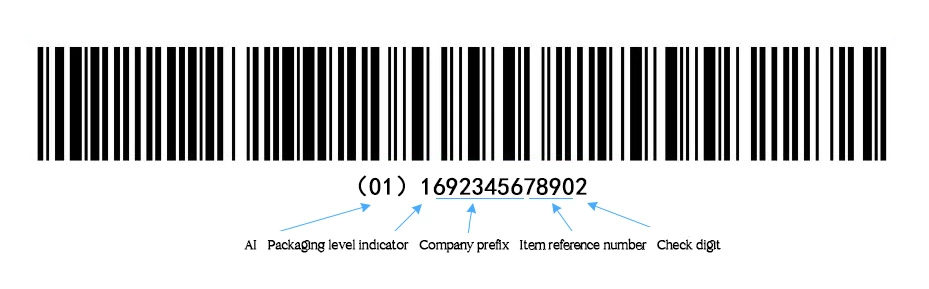
- Application Identifiers (AI): The GS1 Application Identifiers give meaning to the data within a barcode. GS1 Barcode Data Identifiers can vary in length from 2-4 digits. The AI is usually shown in brackets.
- Packaging level indicator:Only suitable for GTIN-14. It can be any number from 1 to 8, indicating different levels of product packaging. The indicators have no specific meaning and do not have to be used in order. Note: GTIN, Global trade item number. GTIN has 4 different code structures, among which GTIN-14 is a 14-digit number used to identify trade items at various packaging levels.
- Company prefix: A GS1 Company Prefix is a number that is unique to each member of GS1 and identifies you as the owner of the brand. It is a building block for every UDI barcode that you will create.
- Item reference number: The owner allocates it according to relevant standards. The manufacturer should follow the meaningless coding principle when allocating commodity item codes, that is, each digit in the item reference number neither indicates the classification nor any specific information.
- Check digit: It follows the standard algorithm, which is specified by UDI standard.
(2) PI generally include: batch / lot number, expiry date, and production date.
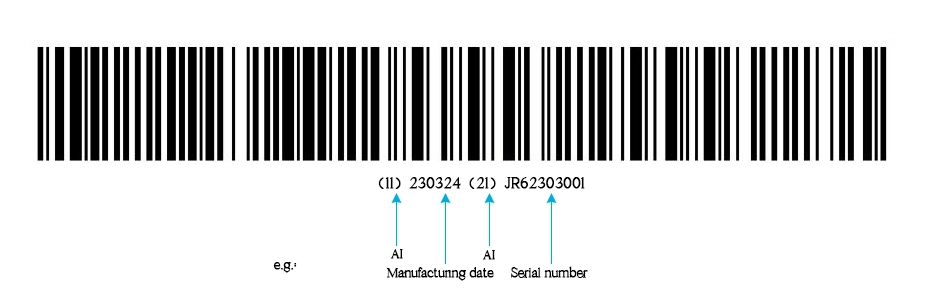
Due to the differences in the use risks and regulatory traceability requirements of medical devices, UDI can be represented by DI alone or by DI+PI. For example:
- Only DI included. Trace back to medical device model.
- DI + PI (production date/expiry date, lot number). Trace back to batch of medical devices.
- DI + PI (production date/expiry date, serial number). Trace back to medical devices.
3. UDI Carrier
UDI Carrier: Linear barcode, 2D barcodes, RFID.

4. What is Basic-UDI?
Basic-UDI: is to be used in the EU as the primary identifier of the device model, assigned at the device unit of use. It is not applied on the devices. It is the main key for records in the UDI Database and is referenced in relevant certificates and EU declarations of conformity.
UDI is a key tool to ensure transparency and visibility in the production, operation and use of medical devices. It can significantly improve product traceability to ensure patient safety. The implementation of UDI has become an indispensable task. JERRY Medical has actively carried out and completed the implementation of UDI to ensure more greater safety and convenience for every user.
PRODUCT LIST
CATEGORY

